Eliquis Recall Over Mislabeling Prompts Dosage Concerns
Bristol-Myers Squibb has issued a voluntary recall relating to Eliquis, the company’s blockbuster blood-thinning drug. The lot in question has been recalled due to an incorrectly printed label stating the package contains 5 mg tablets. Its actual content is half that quantity.
The recall details cover a lot that was distributed to wholesalers and retailers back in February. No injuries or negative health events have been recorded as yet, but the consequences of underdosing this medication are significant enough that Bristol-Myers Squibbs chose to formally pull back the mislabeled product.
Specifically, the risks associated with a lower-than-intended dosage come in the form of blood clots that continue to form, despite the patient believing they have taken the prescribed amount.
Over an extended period, this insufficient quantity could result in stroke and death.
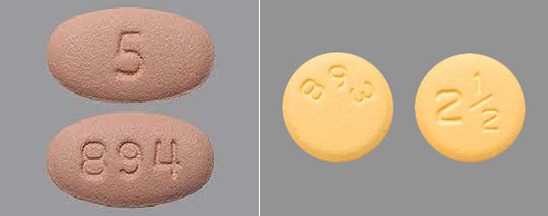
While the recall is primarily aimed at distributors so that the mislabeled drug can be picked up before it gets into the hands of those taking Eliquis, the information campaign extends to make patients aware of the problem. If you take this drug, be sure to check the pills before you take them. Compare the images below to confirm whether you are taking the 2.5 mg pill, which is yellow and round, or the higher dosage of 5 mg, where the pill is pink and oval-shaped.
Deep vein thrombosis (DVT) is a common complaint of patients who are prescribed Eliquis. For these patients, underdosing is a limited concern as the symptoms can usually be reversed by treatment.
Patients with atrial fibrillation complaints are most at risk from the increased potential for blood clots to form. The same is true for people taking the drug to treat complaints in the legs and lungs, as well as patients who have recently undergone hip or knee surgery.
As with all recalls and safety alerts, education is key. The chance of the mislabeled lot getting into the hands of patients who are not made aware of the risk is unlikely, but safety and awareness must come first.
Underdosing a drug can be almost as damaging as overdosing in certain circumstances. If a drug is being prescribed to aggressively hold back a potentially life-threatening health condition, receiving too little of it elevates the risk of that condition developing.
This is why even a small Eliquis recall is a major announcement and should be immediately recognized by any patient taking the drug.